Vanadium »
PDB 3omx-4zg4 »
4qih »
Vanadium in PDB 4qih: The Structure of Mycobacterial Glucosyl-3-Phosphoglycerate Phosphatase RV2419C Complexes with VO3
Enzymatic activity of The Structure of Mycobacterial Glucosyl-3-Phosphoglycerate Phosphatase RV2419C Complexes with VO3
All present enzymatic activity of The Structure of Mycobacterial Glucosyl-3-Phosphoglycerate Phosphatase RV2419C Complexes with VO3:
3.1.3.70;
3.1.3.70;
Protein crystallography data
The structure of The Structure of Mycobacterial Glucosyl-3-Phosphoglycerate Phosphatase RV2419C Complexes with VO3, PDB code: 4qih
was solved by
W.H.Zhou,
Q.Q.Zheng,
D.Q.Jiang,
W.Zhang,
Q.Q.Zhang,
J.Jin,
X.Li,
H.T.Yang,
N.Shaw,
Z.Rao,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 35.01 / 2.30 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 46.312, 82.762, 131.319, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.7 / 24.5 |
Vanadium Binding Sites:
The binding sites of Vanadium atom in the The Structure of Mycobacterial Glucosyl-3-Phosphoglycerate Phosphatase RV2419C Complexes with VO3
(pdb code 4qih). This binding sites where shown within
5.0 Angstroms radius around Vanadium atom.
In total 2 binding sites of Vanadium where determined in the The Structure of Mycobacterial Glucosyl-3-Phosphoglycerate Phosphatase RV2419C Complexes with VO3, PDB code: 4qih:
Jump to Vanadium binding site number: 1; 2;
In total 2 binding sites of Vanadium where determined in the The Structure of Mycobacterial Glucosyl-3-Phosphoglycerate Phosphatase RV2419C Complexes with VO3, PDB code: 4qih:
Jump to Vanadium binding site number: 1; 2;
Vanadium binding site 1 out of 2 in 4qih
Go back to
Vanadium binding site 1 out
of 2 in the The Structure of Mycobacterial Glucosyl-3-Phosphoglycerate Phosphatase RV2419C Complexes with VO3
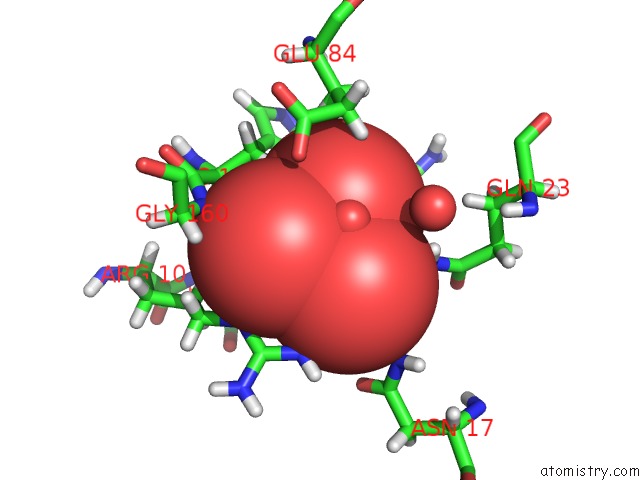
Mono view
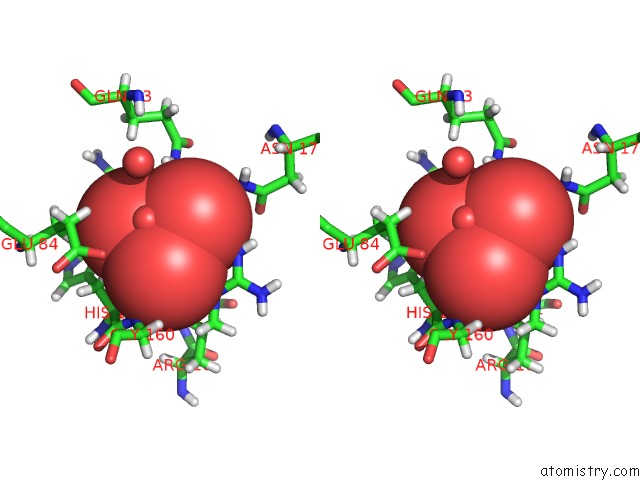
Stereo pair view

Mono view

Stereo pair view
A full contact list of Vanadium with other atoms in the V binding
site number 1 of The Structure of Mycobacterial Glucosyl-3-Phosphoglycerate Phosphatase RV2419C Complexes with VO3 within 5.0Å range:
|
Vanadium binding site 2 out of 2 in 4qih
Go back to
Vanadium binding site 2 out
of 2 in the The Structure of Mycobacterial Glucosyl-3-Phosphoglycerate Phosphatase RV2419C Complexes with VO3
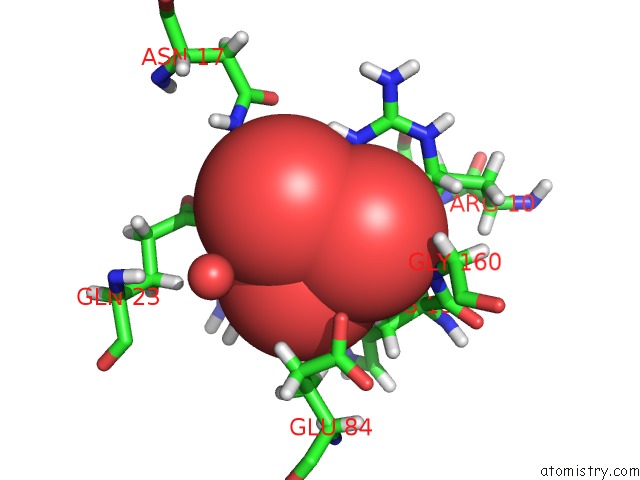
Mono view
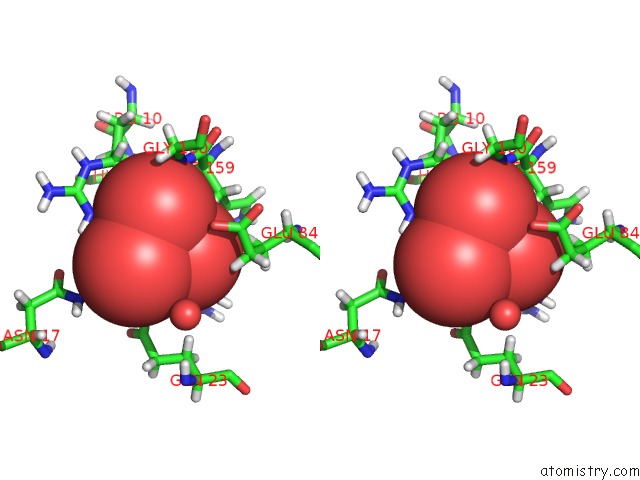
Stereo pair view

Mono view

Stereo pair view
A full contact list of Vanadium with other atoms in the V binding
site number 2 of The Structure of Mycobacterial Glucosyl-3-Phosphoglycerate Phosphatase RV2419C Complexes with VO3 within 5.0Å range:
|
Reference:
Q.Zheng,
D.Jiang,
W.Zhang,
Q.Zhang,
Q.Zhao,
J.Jin,
X.Li,
H.Yang,
M.Bartlam,
N.Shaw,
W.Zhou,
Z.Rao.
Mechanism of Dephosphorylation of Glucosyl-3-Phosphoglycerate By A Histidine Phosphatase J.Biol.Chem. V. 289 21242 2014.
ISSN: ISSN 0021-9258
PubMed: 24914210
DOI: 10.1074/JBC.M114.569913
Page generated: Tue Aug 19 08:11:02 2025
ISSN: ISSN 0021-9258
PubMed: 24914210
DOI: 10.1074/JBC.M114.569913
Last articles
W in 7Z5JW in 7ZPH
W in 7ZPW
W in 6Y7P
W in 7ZCJ
W in 7RCJ
W in 7XQW
W in 7VW6
W in 7T5A
W in 7OWZ